Treatment of Newly Diagnosed Myeloma (excluding t-cell redirection therapy)
Category: Treatment of Newly Diagnosed Myeloma (excluding t-cell redirection therapy)
Autologous peripheral blood hematopoietic stem cell transplantation on the treatment of multiple myeloma: a single-center clinical analysis on PFS
(PA-458) Autologous Peripheral Blood Hematopoietic Stem Cell Transplantation on the Treatment of Multiple Myeloma: A Single-center Clinical Analysis on PFS

Hailong Yuan
Chief Physician
Hematology Center, The First Affiliated Hospital of Xinjiang Medical University
Introduction:
Since the launch of intravenous melphalan (Mel) in China in 2019, our transplantation center has adopted high-dose intravenous Mel as a conditioning regimen, treating 50 consecutive newly diagnosed MM patients receiving ASCT, achieving good clinical efficacy. This study retrospectively analyzed the clinical characteristics and PFS of these 50 MM patients after HDM-ASCT, and preliminarily discussed the impact of different risk levels, response degrees, and minimal residual disease (MRD) on PFS. Our findings may provide more clinical evidence for MM patients before and after ASCT about the importance of MRD monitoring and the impact of the degree of remission on PFS。
Methods:
A retrospective study was conducted on 50 consecutive MM patients who underwent HDM-ASCT. The PFS was the primary outcome, while the treatment efficacy and MRD status after ASCT served as the secondary outcomes.
Results:
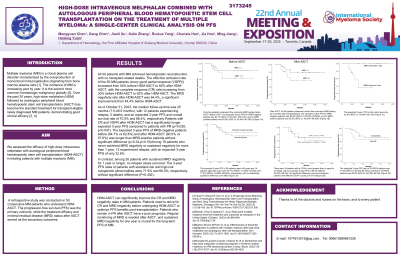
All 50 patients with MM achieved hematopoietic reconstruction, with no transplant-related deaths. The effective remission rate of the 50 MM patients (≥very VGPR)increased from 54% before HDM-ASCT to 82% after HDM-ASCT, with the CR rate increasing from 30% before HDM-ASCT to 62% after HDM-ASCT. The MRD negativity rate after HDM-ASCT was 64.4%, a significant improvement from 44.4% before HDM-ASCT. As of October 31, 2023, the median follow-up time was 25 months (7.5-49.5 months), with 12 patients experiencing relapse, 5 deaths, and an expected 3-year PFS and overall survival rate of 70.5% and 88.4%, respectively.Patients with CR and VGPR after HDM-ASCT had a significantly longer expected 3-year PFS compared to patients with PR. The expected 3-year PFS of MRD-negative patients before (84.7% vs 62.8%) and after HDM-ASCT (80.5% vs 57.6%) was longer than MRD-positive patients,without significant difference.Among 18 patients who never achieved MRD negativity or sustained negativity for more than 1 year, 12 experienced relapse, with an expected 3-year PFS of only 32.6%. In contrast, among 26 patients with sustained MRD negativity for 1 year or longer, no relapse cases occurred. The 3-year PFS rates of patients with standard-risk and high-risk cytogenetic abnormalities were 71.9% and 66.0%, respectively, without significant difference.
Conclusions:
HDM-ASCT can significantly improve the CR and MRD negativity rates in MM patients. Patients need to strive for CR and MRD negativity before undergoing HDM-ASCT to optimize PFS benefits post-transplantation. Patients who remain in PR after ASCT have a poor prognosis. Regular monitoring of MRD is needed after ASCT, and sustained MRD negativity for one year is crucial for the long-term PFS of MM.
