Imaging, QoL and Patient-Reported Outcome and Supportive Care
Category: Imaging, QoL and Patient-Reported Outcome and Supportive Care
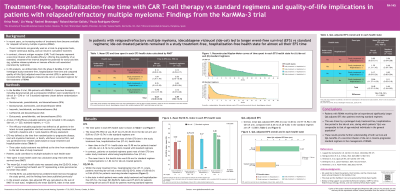
Treatment-Free Hospitalization-Free Time With CAR T-Cell Therapy Versus Standard Regimens and Quality of Life Implications in Patients With Relapsed/Refractory MM: Findings From the KarMMa-3 Trial
(PA-145) Treatment-Free Hospitalization-Free Time With CAR T-Cell Therapy Versus Standard Regimens and Quality of Life Implications in Patients With Relapsed/Refractory MM: Findings From the KarMMa-3 Trial

Krina K. Patel, MD, MSc (she/her/hers)
Associate Professor
Department of Lymphoma and Myeloma, The University of Texas MD Anderson Cancer Center
Introduction: An increasing number of treatments have become available for relapsed/refractory multiple myeloma (RRMM). They are generally treat-to-progression treatments, require continuous dosing and can result in cumulative toxicities. In contrast, CAR T-cell therapies represent a one-time infusion and offer the potential for a treatment-free interval despite potential early onset toxicities. This study utilized data from the KarMMa-3 trial to investigate treatment-free hospitalization-free time and compared quality of life (QoL)-adjusted event-free survival (EFS) in patients with RRMM treated with idecabtagene vicleucel (ide-cel) versus standard regimens, accounting for hospitalizations due to infusion, serious adverse events, and other factors.
Methods: EFS, defined as the time from randomization to progressive disease, next anti-myeloma treatment, or death, was partitioned into 4 health states based on study treatment and hospitalization status. Patients could contribute multiple episodes in each health state. Time spent in each health state was calculated using restricted mean survival time (RMST). The mean QoL in each health state was assessed using the EQ-5D index, wherein 1 represents full health and 0 represents a state perceived as no better than death. QoL-adjusted EFS, defined as the sum of RMST in each state weighted by the mean EQ-5D index in that state, was reported.
Results:
348 efficacy-evaluable patients were included in the analysis (ide-cel, n=225; standard regimen, n=123). The mean EFS (95% CI) was 18 mo (16, 20) in the ide-cel and 9 mo (7, 11) in the standard regimen arms. Patients treated with ide-cel spent most of their EFS time in a treatment-free hospitalization-free state, with an average (95% CI) of 16 mo (14-18) compared with 0.16 mo (0.07-0.28) for those on standard regimens. Patients on standard regimens spent much of their EFS time on study treatment while being hospitalization-free, with a mean of 9 mo (7-10) versus 1.84 mo (1.76-1.94) for ide-cel patients. Within the treatment-free hospitalization-free state, ide-cel patients reported a mean EQ-5D index (SD) of 0.80 (0.14) compared with 0.66 (0.05) for standard regimen patients. In the state where patients were under treatment and hospitalization-free, the mean EQ-5D index (SD) was 0.75 (0.20) for ide-cel and 0.75 (0.17) for standard regimen patients. Overall, the QoL-adjusted EFS (95% CI) was 14 mo (13-16) in the ide-cel arm compared with 7 mo (5-8) in the standard regimen arm (P< 0.0001).
Conclusions: Patients receiving ide-cel experienced significantly longer QoL-adjusted EFS, driven by a prolonged treatment-free and hospitalization-free state, as well as improved QoL in this state, during which their QoL matched that of the general population of the same age. These results provide further understanding of both survival and QoL benefits of a one-time infusion of ide-cel versus the treat-to-progression standard regimen.
