Cellular and T cell engager Immunotherapy
Category: Cellular and T cell engager Immunotherapy
Phase 2 Study of Talquetamab (Tal) + Teclistamab (Tec) in Patients (pts) With Relapsed/Refractory Multiple Myeloma (RRMM) and Extramedullary Disease (EMD): RedirecTT-1
(PA-060) Phase 2 Study of Talquetamab (Tal) + Teclistamab (Tec) in Patients (pts) With Relapsed/Refractory Multiple Myeloma (RRMM) and Extramedullary Disease (EMD): RedirecTT-1

Saad Z. Usmani, MD, MBA, FACP, FASCO
Chief Attending, Myeloma Service
Memorial Sloan Kettering Cancer Center
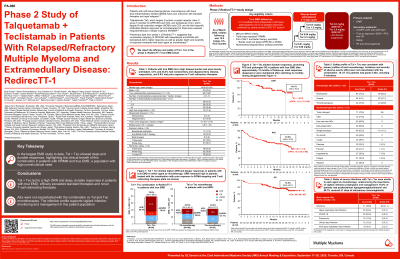
Tal (anti-GPRC5D) and Tec (anti-BCMA) are bispecific antibodies (BsAbs) approved as monotherapies for triple-class exposed (TCE) RRMM. In pts with EMD, ORR was 41–48% with Tal and 36% with Tec alone. We report the efficacy and safety of Tal + Tec in pts with EMD in the phase 2 RedirecTT-1 EMD cohort (NCT04586426).
Methods:
Pts had TCE RRMM and EMD (≥1 nonradiated soft tissue plasmacytoma noncontiguous with bone ≥2 cm in 1 dimension with or without paraskeletal plasmacytomas). Nonsecretory/oligosecretory disease permitted. Prior CAR-T (≤20% of pts) and non-BCMA/-GPRC5D BsAb therapy permitted. Pts received Tal 0.8 mg/kg Q2W + Tec 3.0 mg/kg Q2W, with step-up doses; pts could switch to Q4W dosing at investigator’s discretion after cycle 6 or after cycle 4 with confirmed ≥VGPR. Response was assessed by IRC per IMWG criteria; EMD response was assessed by PET-CT scans.
Results: As of March 2025, 90 pts received Tal + Tec (median follow-up 12.6 mo [range 0.5–19.5]). Median age 65 yrs; 22% had high-risk cytogenetics, 39% had nonsecretory/oligosecretory disease, and median number of plasmacytomas noncontiguous with bone was 2 (range 1–14). Median prior LOT was 4: 84% triple-class refractory, 36% penta-drug refractory, 20% prior anti-BCMA CAR-T therapy, and 9% prior BsAbs. ORR (95% CI) was 79% (69.0–86.8), with ≥CR 52%; ORR was 83% (58.6–96.4; n=15/18) in anti-BCMA CAR-T–exposed pts and 75% (34.9–96.8; n=6/8) in BsAb-exposed pts. 9-mo DOR, PFS, and OS were 75%, 64%, and 80%, respectively. Most responders ( >90%) deepened or maintained response after switching to Q4W dosing. Grade (gr) 3/4 AEs occurred in 78 (87%) pts. CRS occurred in 70 (78%) pts (all gr 1/2). ICANS occurred in 11 (12%) pts (gr 3, 1%; gr 4, 1%; gr 5, 0%). Neutropenia was the most common gr 3/4 AE (n=56, 62%). Taste changes (n=71, 79%), skin (n=62, 69%), and nail (n=50, 56%) were all gr 1/2, and rash (n=26, 29%) was mostly gr 1/2. Infections occurred in 71 (79%) pts (gr 3/4, 37%); 88% of gr 3/4 infections occurred within the first 6 mo. 63 (70%) pts had posttreatment hypogammaglobulinemia. 78 (87%) pts received ≥1 dose of intravenous IgG. 8 (9%) pts discontinued Tal + Tec due to AEs; 5 due to gr 5 AEs (COVID-19 pneumonia, Klebsiella sepsis, aspiration, respiratory failure, euthanasia) and 3 due to non–gr 5 AEs. 2 pts discontinued Tal only due to non–gr 5 AEs. No pts discontinued Tec only. 10 pts had gr 5 AEs (5 infections); 5 were drug related.
Conclusions: With 90 pts, the phase 2 cohort of RedirecTT-1 is the largest dedicated EMD study to date. Tal + Tec led to a high ORR and deep, durable responses; efficacy exceeded standard therapies, including BsAbs alone, and was comparable to CAR-T in pts with RRMM with EMD. No new safety signals were identified, including no exacerbated Tal or Tec AEs. These data highlight the clinical benefit of dual antigen targeting with the combination of Tal + Tec in pts with EMD, a population with high disease burden and significant unmet need.
