Myeloma Novel Drug Targets and agents
Category: Myeloma Novel Drug Targets and agents
Safety Profile and Toxicity Comparison of Bispecific Antibodies in Relapse Refractory Multiple Myeloma: A Systematic Review of Clinical Trials
(PA-294) Safety Profile and Toxicity Comparison of Bispecific Antibodies in Relapse Refractory Multiple Myeloma: A Systematic Review of Clinical Trials

Shahzad Raza, MD (he/him/his)
Oncologist
Taussig Cancer Institute, Cleveland Clinic
Bispecific antibodies (BsAbs) have emerged as a promising therapeutic strategy for patients with relapsed or refractory multiple myeloma (MM). However, their clinical use is often limited by a distinct and potentially severe toxicity profile, necessitating a deeper understanding of safety outcomes across BsAb targets.
Methods:
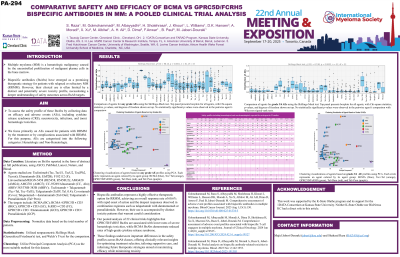
We performed a comprehensive pooled analysis of clinical trials evaluating BsAbs from both full-text publications and conference abstracts available up to April 2025. BsAbs were categorized based on their target antigens into two groups: B-cell maturation antigen (BCMA)-directed BsAbs and those targeting GPRC5D or FcRH5. To compare safety profiles across agents, Welch’s t-test was applied. Principal component analysis (PCA) was also used to explore clustering patterns and identify similarities and differences in adverse events (AEs).
Results:
We analyzed 22 trials involving 2,374 patients with MM from early 2023 to April 2025. Among these, 1,276 patients received BCMA BsAbs, 841 patients were treated with GPRC5D/FcRH5 BsAbs, 157 received teclistamab + talquetamab (Tal), 65 patients received Tal+daratumumab (Dara), and 35 patients received Tal+pomalidomide (Pom). The overall response rate (ORR) was 60.57% in patients with a median follow-up of 11.83 months and a median of 5 prior lines of therapy. The Tal-based combinations (Tal+Dara/Pom) showed the highest efficacy, with an ORR of 81% and the highest rates of CR/sCR (19.62%) and VGPR (25%). Among all-grade hematologic AEs, neutropenia occurred in 40.4%, anemia in 39.2%, thrombocytopenia in 21.4%, lymphopenia in 19.2%, infections in 45.8%, and cytokine release syndrome (CRS) in 65%. For grade 3/4 AEs, infections occurred in 20.3%, CRS in 1.5%, neutropenia in 35.2%, anemia in 24.5%, thrombocytopenia in 13.5%, and lymphopenia in 17.7%. CRS and the need for tocilizumab were significantly more frequent with BCMA BsAbs vs GPRC5D/FcRH5 BsAbs, P< 0.024. Skillings Mack (Generalized Friedman's) findings emphasized substantial distinctions between BCMA and GPRC5D/FcRH5XCD3 in both overall and severe grade 3/4 AEs (p< 0.0002). PCA revealed agents with all grades and grade 3/4 showed similar clustering patterns except for three agents. Fatal AEs occurred in 12.55% of patients, primarily due to progressive disease (3.58%) or treatment-related toxicities (3.88%), with a higher rate of AE-related mortality observed in the BCMA-targeted BsAb group.
Conclusions: The use of BsAbs in MM has demonstrated excellent efficacy; however, these agents have been linked to a unique AE profile. BCMA BsAbs were associated with less hematologic toxicity, 22.4% (grade 3/4: 17.4%) vs 30.6% (grade 3/4: 22%) for GPRC5D/FcRH5, and BCMA BsAbs were associated with lower CRS rates, 56.8% (grade 3/4: 1.3%) vs 73.1% (grad 3/4: 1.7%) for GPRC5D/FcRH5. This is important information for treatment selection and mitigation strategy development aiming to optimize patient outcomes.
