Myeloma Genomics and Microenvironment and immune profiling
Category: Myeloma Genomics and Microenvironment and immune profiling
The m5C-binding protein YB-1 modulates proteasome inhibitors resistance in multiple myeloma via epigenetic modification
(PA-280) The m5c-binding Protein YB-1 Modulates Proteasome Inhibitors Resistance in Multiple Myeloma via Epigenetic Modification

Qiqi Shen
MD
Bone Marrow Transplantation Center, The First Affiliated Hospital, Zhejiang University, School of Medicine
Introduction:
Multiple myeloma (MM) is a malignant proliferative disorder of plasma cells, and resistance to proteasome inhibitors (PIs) represents a major obstacle in MM treatment. Emerging evidence suggests that 5-methylcytosine (m5C) plays a pivotal role in driving MM drug resistance, although the underlying mechanisms remain unclear. Notably, previous studies have established that Y-box binding protein 1(YB-1) functions as a cytoplasmic m5C reader, regulating RNA metabolism through diverse mechanisms.
Methods: Bone marrow samples from MM patients and peripheral blood mononuclear cells from healthy donors were collected after obtaining informed consent. CD138+ cells were isolated via positive selection using CD138-conjugated microbeads. The human myeloma cell lines were transfected with short hairpin RNA lentiviral particles in order to obtain stable cell lines with different YB-1 expression levels. Cell viability was evaluated using flow cytometry and cell counting kit-8 assays.
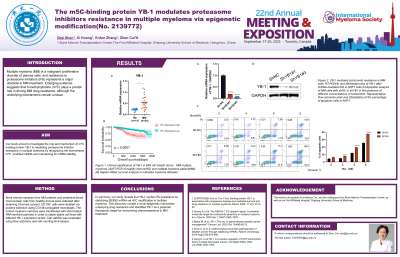
Results: Here, we found that YB-1 is highly expressed in CD138+ cells isolated from MM patients compared to healthy donors and is associated with poor prognosis. In vitro experiments suggested that YB-1 knockdown could greatly trigger the apoptosis of MM cells and induce G2 phase arrest under the treatment of PIs. We restored YB-1 expression by overexpressing wild-type (WT) YB-1 or the binding deficient mutant YB-1-Mut(W65F) on the basis of YB-1 knockdown. Subsequently, we observed that the restoration of WT-YB1, but not YB-1-Mut(W65F), substantially rescued the survival of MM cells under PIs treatment, indicating that YB-1 regulates PIs resistance in MM in a m5C-dependent manner. To identify YB-1-mediated downstream effectors modulating MM PIs resistance, we conducted multiple high-throughput sequencing analyses (Bis-seq, RIP-seq, and RNA-seq) and obtained three potential downstream targets (SESN2, ZFP36, TTYH3). Further analysis of MM patient gene expression profiles suggested a stronger correlation between YB-1 and SESN2. We conducted an RNA decay assay in MM cells and our data showed that YB-1 maintains SESN2 mRNA stability by binding to its m5C methylation sites. SESN2 is a highly conserved stress-induced protein that protects cells from external factors such as stress and autophagy. Based on survival analysis, we found that high expression of SESN2 is associated with poor prognosis of MM patients. Furthermore, in vitro phenotypic assays showed that the depletion of SESN2 significantly enhanced the killing effect of PIs on MM cells, while the supplementation of SESN2 substantially rescued the PIs sensitivity of MM cells resulting from YB-1 deficiency.
Conclusions:
In summary, our study reveals that YB-1 confers PIs resistance by stabilizing SESN2 mRNA via m5C modification in multiple myeloma. This discovery unveils a novel epigenetic mechanism underlying drug resistance and identifies YB-1 as a potential therapeutic target for overcoming chemoresistance in MM treatment.
