Treatment of Newly Diagnosed Myeloma (excluding t-cell redirection therapy)
Category: Treatment of Newly Diagnosed Myeloma (excluding t-cell redirection therapy)
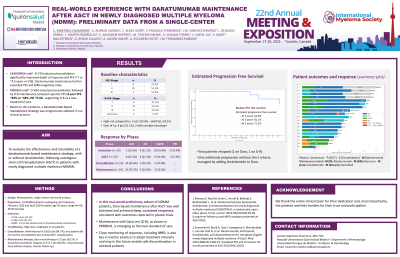
Real-World Experience with Daratumumab Maintenance After ASCT in Newly Diagnosed Multiple Myeloma (NDMM): Preliminary Data From a Single-Center
(PA-409) Real-World Experience with Daratumumab Maintenance After ASCT in Newly Diagnosed Multiple Myeloma (NDMM): Preliminary Data From a Single-Center

Carmen Martínez Chamorro, MD, PhD (she/her/hers)
Hematologist
Hospital Quirónsalud Madrid
Introduction:
The addition of daratumumab to first-line treatment regimens for newly diagnosed multiple myeloma (NDMM) has shown superior depth of response and progression-free survival (PFS) in phase III trials such as CASSIOPEIA and PERSEUS. These studies established daratumumab-based maintenance, either as monotherapy or in combination with lenalidomide (D-R), as a standard post-transplant approach. Based on this evidence, a daratumumab-based maintenance strategy was progressively adopted in our routine clinical practice.
Methods:
A retrospective real-world analysis was conducted including 19 patients with NDMM who received maintenance with daratumumab, either as monotherapy or combined with lenalidomide, following autologous stem cell transplantation (ASCT). Median age at diagnosis was 59 years (range: 44–69), and 36.8% were female. At diagnosis, 31.6% of patients were ISS stage I, 52.6% stage II, and 15.8% stage III. R-ISS stages were I (31.6%), II (52.6%), III (10.5%), and unknown (1 patient). High-risk cytogenetics [del(17p) or t(4;14)] were present in 10.5% of cases; 21.1% showed gain of 1q, with two cases also presenting complex karyotype.
All patients underwent daratumumab-based induction (D-VTd: 73.7%, D-VRd: 21.1%, DVMP: 5.3%) and received melphalan conditioning prior to ASCT. Post-transplant consolidation was given in 94.7% of cases. Maintenance therapy consisted of daratumumab alone (63.2%) or in combination with lenalidomide (36.8%). Five patients discontinued treatment without relapse.
Results:
Response deepened across treatment phases. Post-induction, 10.5% achieved sCR, 21.1% CR, and 52.6% VGPR. After ASCT, 42.1% reached sCR. Following consolidation and during maintenance, the sCR rate increased to 55.6% and 73.7%, respectively. Estimated PFS was 93.6% at 2 years, 81.1% at 3 years, and 71.2% at 5 years. Two patients relapsed (one on daratumumab, one on D-R), and one case showed progression without strict criteria, managed by adding lenalidomide.
Conclusions:
This real-world cohort shows that daratumumab-based maintenance post-ASCT is well tolerated and highly effective. Most patients achieved deep responses, with sustained PFS over time. Combination with lenalidomide (D-R), as used in PERSEUS, offers a clinically relevant option and allows for adjustment of treatment intensity based on individual response. Future clinical trials may consider evaluating treatment discontinuation in standard-risk patients with sustained MRD negativity.
