Cellular and T cell engager Immunotherapy
Category: Cellular and T cell engager Immunotherapy
Real-World Outcomes of Equecabtagene Autoleucel, the First Fully Human BCMA-Targeted CAR-T Therapy, in 150 Patients with Multiple Myeloma (MM): A Multicenter Experience from China
(PA-065) Real-World Outcomes of Equecabtagene Autoleucel, the First Fully Human BCMA-Targeted CAR-T Therapy, in 150 Patients with Multiple Myeloma (MM): A Multicenter Experience from China

Jin Lu, MD
Professor
Peking University People’s Hospital, Peking University Institute of Hematology, National Clinical Research Center for Hematologic Disease,Beijing,China; Department of Hematology, Fu Xing Hospital, Capital Medical University, Beijing, China.
Equecabtagene Autoleucel (Eque-cel), a fully human BCMA-targeted CAR-T therapy, received NMPA approval in June 2023 for adults with relapsed/refractory multiple myeloma (RRMM) after ≥3 prior therapies. Building on the FUMANBA-1 trial’s demonstration of durable efficacy and safety in heavily pretreated RRMM, this study presents the first post-approval real-world analysis of Eque-cel in China, offering clinical insights for its use.
Methods:
We conducted analysis on data from 150 patients who received infusion therapy across 48 centers between June 2023 and August 31, 2024.All patients received a single infusion of Eque-cel at the dose of 1.0 x 106 CAR-T cells/kg. Responses were evaluated per IMWG criteria, and the ORR was defined as at least ≥PR.
Results:
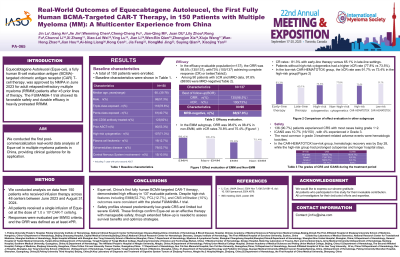
All 150 patients underwent leukapheresis followed by Eque-cel infusion. Median age was 60 years (range: 35-78), with 33.3% aged ≥65. Disease characteristics included high-risk cytogenetics (71.3%, 107/150), plasma cell leukemia (12.7%, 19/150), extramedullary myeloma (52.7%, 79/150), and CNS involvement (10%, 15/150).
The median prior therapy lines were 3 (range:1-9), with 78.6% (118/150) triple-class exposed, 40.7% (61/150) penta-exposed, 82% (123/150) anti-CD38 antibody-treated, and 53.3% (80/150) with prior ASCT(including 7 with prior second ASCT). The CAR-HEMATOTOX classifier stratified 82 patients into low- (n=12) and high-risk (n=70) groups.
In the efficacy-evaluable population (n=137; excluding 10 deaths, 1 lost to follow-up, and 2 missing data), the overall response rate (ORR) was 98.5% (135/137), with 73% (100/137) achieving complete response (CR) or better. Among 90 patients with ≥CR and MRD data, 97.8% (88/90) were MRD-negative.
In the EMM subgroup, ORR was 98.6% vs 98.4% in non-EMM, with ≥CR rates 70.8% and 75.4% respectively. The CR rates showed significant variation between treatment lines, with early-line therapy achieving 81.3% CR vs 65.1% CR in late-line settings.Patients without high-risk cytogenetics had a higher ≥CR rate (77.8% vs 73.5%). In the low-risk CAR-HEMATOTOX group, the ≥CR rate was 91.7% vs 73.4% in the high-risk group.
In the available dataset, CRS occurred in 90.7% (136/150). Most cases were Grade 1 or 2, while higher - severity cases were more prevalent in high - risk patients. ICANS was 10.7% (16/150) , with 4% experienced ≥ Grade 3. In the CAR-HEMATOTOX low-risk group, hematologic recovery was by Day 28, while the high-risk group had prolonged cytopenias and longer hospital stays.
Conclusions:
Eque-cel, China’s first fully human BCMA-targeted CAR-T therapy, demonstrated high efficacy in 137 evaluable patients. Despite high-risk features including EMM(52.7%), PCL(12.7%), and CNS infiltration (10%), outcomes aligned with the pivotal FUMANBA-1 trial. Safety profiles showed predominantly low-grade CRS and limited but severe ICANS. These findings confirm Eque-cel as an effective therapy with manageable safety, though extended follow-up is needed to assess survival benefits and optimize strategies.
