Treatment of Newly Diagnosed Myeloma (excluding t-cell redirection therapy)
Category: Treatment of Newly Diagnosed Myeloma (excluding t-cell redirection therapy)
Efficacy and Safety of Bortezomib, Pomalidomide and Dexamethasone (VPD) in Newly Diagnosed Multiple Myeloma: Single-arm, Phase-II Investigator Initiated Prospective Clinical Trial
(PA-406) Efficacy and Safety of Bortezomib, Pomalidomide and Dexamethasone (VPD) in Newly Diagnosed Multiple Myeloma: Single-arm, Phase-II Investigator Initiated Prospective Clinical Trial

Jeevan Kumar, MD, DrNB Hematology (he/him/his)
Senior Consultant
Tata Medical Center, Kolkata, India
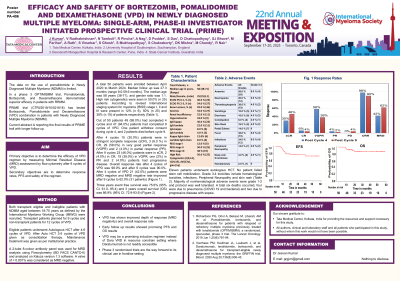
Introduction: The data on the use of pomalidomide in Newly Diagnosed Multiple Myeloma (NDMM) is limited. Primary objective of this study was to determine the efficacy of VPD regimen by measuring Minimal Residual Disease (MRD) by flow-cytometry after 9 cycles of VPD. Current abstract is reporting the final results of PRIME trial with longer follow up.
Methods: Both transplant eligible and ineligible patients with NDMM aged between 18-70 years as defined by the IMWG were recruited. Transplant patients planned for 9 cycles and non-transplant patients for 12 cycles of VPD. Eligible patients underwent Autologous HCT after 4-6 cycles of VPD. After Auto HCT 3-5 cycles of VPD given as consolidation therapy. Maintenance treatment was given as per institutional practice. A 2-tube 8-colour antibody panel was used for MRD analysis using Flowcytometry (BD FACS CANTO-II) and analysed on Kaluza version 1.3 software. A valve of < 0.001% was considered as MRD negative.
Results:
A total 50 patients were enrolled between April 2020 to March 2024. Median follow up was 27.3 months (range 9.0-59.6 months). The median age was 58 years (38-71), and gender ratio was 1:1. High risk cytogenetics were seen in 50.0% (n 25) patients. According to revised international staging system for myeloma (RISS) stage I, II and III were present in 12% (n 6), 50% (n 25) and 38% (n 19) of patients respectively.
Out of 50 patients 49 (98.0%) had completed 4 cycles and 47 (94.0%) patients had completed 9 cycles of VPD. After 4 cycles 15 (30.0%) patients were in stringent complete response (sCR), 3 (6.0 %) patients in CR, 29 (58.0%) in very good partial response (VGPR) and 2 (4.0%) in partial response (PR). After 9 cycles 23 (46.0%) patients were in sCR, 2 (4.0%) in CR, 19 (38.0%) in VGPR, one (2%) in PR and 2 (4.0%) patients had progressive disease. Overall response rate after 4 cycles of VPD was 98.0% and after 9 cycles was 90.0%. After 4 cycles of VPD 21 (42.0%) patients were MRD negative and MRD negative rate improved after 9 cycles to 62.0% (31 patients). Three years event free survival was 75.6% (95% CI: 61.0- 85.4) and 3 years overall survival (OS) was 86.9% (95% CI: 72.9-93.9).
Eleven patients underwent auto HCT. No patient failed stem cell mobilization. Grade 3-4 toxicities include hematological toxicities (10%), infections (10%) and skin rash (4%). Majority of nonhematological adverse events were grade 1 to 2 and protocol was well tolerated. A total six deaths occurred, four were due to pneumonia (COVID 19 and bacterial) and two due to progressive disease with sepsis.
Conclusions: VPD has shown improved depth of response (MRD negativity) and overall response rate. Early follow up results showed promising PFS and OS results. VPD may be a promising induction regimen instead of Dara VRD in resource constrain setting where Daratumumab is not readily accessible. Phase 3 randomized trials are the way forward to its clinical use in frontline setting.
