Imaging, QoL and Patient-Reported Outcome and Supportive Care
Category: Imaging, QoL and Patient-Reported Outcome and Supportive Care
Patient-reported quality of life with linvoseltamab in triple-class exposed patients with relapsed/refractory multiple myeloma: 2-year results from the LINKER-MM1 Phase 1/2 clinical trial
(PA-121) Patient-reported Quality of Life with Linvoseltamab in Triple-class Exposed Patients with Relapsed/refractory Multiple Myeloma: 2-year Results from the LINKER-MM1 Phase 1/2 Clinical Trial

Joshua Richter, MD
Associate Professor
Icahn School of Medicine at Mount Sinai
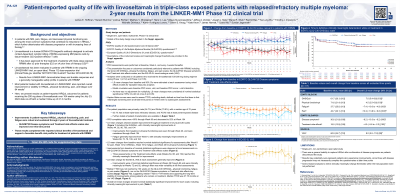
In LINKER-MM1, most patients (pts) with relapsed/refractory multiple myeloma treated with linvoseltamab reported overall improvement or stability in quality of life (QoL), physical functioning (PF), pain, and fatigue over 84 weeks. Here, we present results over 104 weeks.
Methods:
We evaluated 117 pts in LINKER-MM1 who were assigned to receive the 200 mg dose of linvoseltamab. The EORTC QLQ-C30 (global health status [GHS]/QoL, PF, pain, and fatigue) and EQ-5D-3L were conducted at baseline (BL), Week (W) 4, and every 4 weeks thereafter. Least squares (LS) mean change from BL and 95% confidence interval (CI) were estimated at each time point and overall using a mixed effects model for repeated measures; 10- (QLQ-C30) and 12-point (EQ-5D-3L visual analog scale [VAS]) changes were considered clinically meaningful. As there was no adjustment for multiplicity, statistical significance is nominal. Kaplan–Meier curves assessed time to definitive deterioration (TTDD) defined from first dose to first meaningful worsening seen at all later time points or if there were no subsequent assessments.
Results:
Questionnaire completion rates were >80% through W80 and decreased to 52% at W104. LS mean changes showed durable improvement over the treatment duration in key QLQ-C30 scales, including GHS/QoL, with clinically meaningful improvements at W44 (10.0), W92 (12.2), W96 (10.0), W100 (11.9), and W104 (10.1), and EQ-5D-3L VAS, with clinically meaningful improvements at W72 (12.2) and W92 (13.8); pain improvement was meaningful at most time points (W20, −15.6; W24, −13.1; W28, −11.0; W40, −10.1; W52, −13.8; W56, −10.5; W64, −10.0; W68, −12.8; W72, −11.2; W76, −13.4; W88, −14.4; W96, −10.2; W100, −12.7; W104, −11.8). Improvements in PF were seen through W20, and were generally maintained thereafter. Fatigue generally improved from W4 onwards, with clinically meaningful improvements at W68 (−10.1), W72 (−12.0), W76 (−11.6), W100 (−14.0), and W104 (−10.8). LS mean changes from BL reached nominal statistical significance (ie, 95% CI of change did not cross 0) for most weeks after W12 for GHS/QoL, W20 for PF, W16 for fatigue, W8 for pain, and W20 for EQ-5D-3L VAS. Overall LS mean (95% CI) improvement was clinically meaningful for pain (−10.2 [−13.8, −6.6]), and of nominal statistical significance for GHS/QoL (7.0 [5.4, 8.6]), PF (4.5 [2.5, 6.6]), fatigue (−7.4 [−10.0, −4.9]) and EQ-5D-3L VAS (8.0 [5.0, 11.0]). Median TTDD was not reached ( >104 weeks).
Conclusions:
Improvements in pt-reported QoL, PF, pain, and fatigue were robust over 2 years of linvoseltamab treatment, complementing clinical benefits and supporting a favorable benefit–risk profile.
