Cellular and T cell engager Immunotherapy
Category: Cellular and T cell engager Immunotherapy
Value of the treatment-free period following ciltacabtagene autoleucel infusion in patients with relapsed/refractory multiple myeloma: preliminary findings from a qualitative interview study
(PA-103) Value of the Treatment-free Period Following Ciltacabtagene Autoleucel Infusion in Patients with Relapsed/refractory Multiple Myeloma: Preliminary Findings from a Qualitative Interview Study
- JM
Methods:
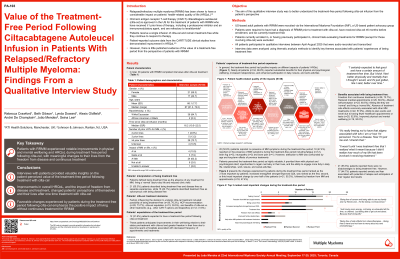
US-based adult patients with RRMM who received cilta-cel ≥6 months before study enrollment and currently treatment free, recruited via the International Myeloma Foundation, participated in semi-structured, qualitative interviews that were audio-recorded and transcribed. Thematic analysis identified key themes associated with patients’ interpretation of “treatment free” and their treatment-free experiences.
Results:
Interviews were completed with 15 patients (9 female/6 male; age range: 53-78; median time since cilta-cel: 13 months 4 days). Patient interpretations of being “treatment free” included freedom from treatment side effects, absence of symptoms, increased freedom of time, and a return to ‘normal’ life. The treatment-free period for most patients was a positive experience with substantial benefits for their HRQOL; “well, I definitely feel better, and I think I'm better off”. Patients reported that they were more able to engage in normal, everyday activities, and notable improvements in their psychological/emotional well-being; 8 patients reported that they no longer ascribed to viewing themselves as a ‘patient’ due to the reduction in medical appointments/absence of continuous treatments; “I don't really see myself as a patient anymore”. Patients highlighted the meaningfulness of increased involvement in family life and strengthening of familial bonds because they were treatment free. Challenges associated with the treatment-free period focused on the fear of potential relapse (n=8) and the resumption of treatment; for many patients, being off continuous therapy represented an important achievement. The most important changes experienced during the treatment-free period included: improved well-being/HRQOL/increased energy (n=10), increased free time (n=8), feeling cancer/disease free (n=7), and freedom from medication/treatment (n=7). For most patients (n=9), the biggest impact of being treatment free was improved psychological wellbeing; “It gave me back enjoyment of life”.
Conclusions:
These initial interviews provide valuable insights on the patient-perceived value of the treatment-free period following cilta-cel treatment for patients with RRMM. Notably, improvements in patients’ overall HRQOL and the impact of freedom from disease and treatment improved patients’ perceptions of themselves and their lives. The changes experienced by patients emphasize the positive impact of living without continuous treatment.
