Treatment of Relapsed/Refractory Myeloma (excluding T-cell redirection therapy)
Category: Treatment of Relapsed/Refractory Myeloma (excluding T-cell redirection therapy)
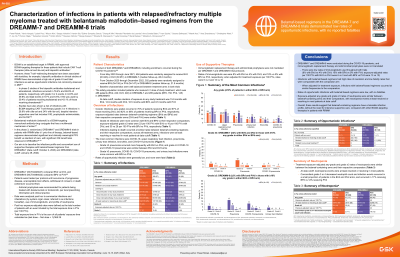
Characterization of Infections in Patients With Relapsed/Refractory Multiple Myeloma (RRMM) Treated With Belantamab Mafodotin (Belamaf)–Based Regimens From the DREAMM-7 and DREAMM-8 Trials
(PA-502) Characterization of Infections in Patients With Relapsed/Refractory Multiple Myeloma (RRMM) Treated With Belantamab Mafodotin (Belamaf)–Based Regimens From the DREAMM-7 and DREAMM-8 Trials

Meral Beksac, MD (she/her/hers)
Professor of Hematology
Istinye University
Methods: DREAMM-7 compared belamaf + bortezomib + dexamethasone (BVd) vs daratumumab + bortezomib + dexamethasone (DVd); DREAMM-8 compared belamaf + pomalidomide + dexamethasone (BPd) vs pomalidomide + bortezomib + dexamethasone (PVd). Data were analyzed post hoc to summarize infections and immunoglobulin use. Post hoc exposure-adjusted rates were defined as total number of pts with an event divided by total exposure time in person-years (PYs; per 100 PYs).
Results:
The DREAMM-7 and DREAMM-8 trials were conducted during the COVID-19 pandemic. Median treatment durations were 15.9, 12.9, 16.5, and 8.5 months with BVd, DVd, BPd, and PVd, respectively. Treatment exposure-adjusted any-grade infection rates were similar between BVd (51) and DVd (55), and lower with BPd (59) vs PVd (73); exposure-adjusted grade ≥3 rates were 23 vs 16 per 100 PYs with BVd vs DVd, respectively, and 35 vs 28 per 100 PYs with BPd vs PVd. Grade 5 infection rates were comparable between arms in both trials. The most common infections, by system organ class (occurring in ≥20% of pts in the BVd or BPd arm) were COVID-19, upper respiratory tract infection and pneumonia. Unadjusted incidence of grade ≥3 pneumonia was higher with BVd/BPd vs that with the comparators. Grade ≥3 COVID-19 was more common with BPd vs PVd but similar between the BVd and DVd arms. At data cutoff, infections were resolved/resolving in most pts. Incidence of “CMV reactivation,” “pneumonia fungal,” “Pneumocystis jirovecii pneumonia,” and “polyomavirus-associated nephropathy,” was low (≤2% each) with BVd and BPd; none were fatal. Rates of immunoglobulin use were 8%, 4%, 18% and 9% with BVd, DVd, BPd and PVd, respectively.
Conclusions:
Despite the conduct of DREAMM-7 and DREAMM-8 during the COVID-19 pandemic, and low immunoglobulin replacement during treatment, infection fatality rates with belamaf-based regimens were low and similar to those with the comparators; infections were resolved/resolving in most pts. Results suggest that belamaf-based regimens have a manageable infection profile in pts with RRMM.
Funding: GSK. Drug-linker technology licensed from Seagen Inc.; monoclonal antibody produced using POTELLIGENT Technology licensed from BioWa
