Myeloma Novel Drug Targets and agents
Category: Myeloma Novel Drug Targets and agents
IBERDOMIDE (IBER) TREATMENT (Tx) ENHANCES MANUFACTURED CHIMERIC ANTIGEN RECEPTOR (CAR) T CELL EXPANSION AND FUNCTIONALITY AND IS IMMUNOSTIMULATORY IN PATIENTS POST-CAR T CELL THERAPY
(PA-307) IBERDOMIDE (IBER) TREATMENT (Tx) ENHANCES MANUFACTURED CHIMERIC ANTIGEN RECEPTOR (CAR) T CELL EXPANSION AND FUNCTIONALITY AND IS IMMUNOSTIMULATORY IN PATIENTS POST-CAR T CELL THERAPY

Michael D. Amatangelo, PhD
Scientific Director, Translational Medicine
Bristol Myers Squibb, Summit, NJ, USA
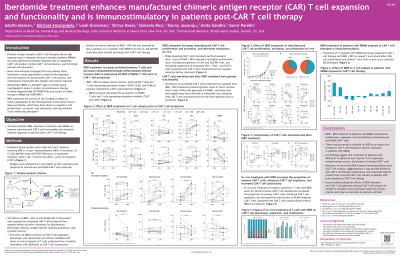
CAR T cell therapies show high response rates in relapsed/refractory multiple myeloma (RRMM), but many patients (pts) produce suboptimal CAR T cells or lack durable responses due to limited cell persistence and functional exhaustion. Tx to improve CAR T cell expansion and functionality, and maintenance Tx to deepen/extend responses are needed. IBER is an oral CELMoD™ agent that induces enhanced degradation of Ikaros and Aiolos compared with IMiD® agents. IBER increases proliferation, activation, and reduces T cell exhaustion. Here, we assess if IBER Tx of pts with RRMM can improve CAR T cell functionality and stimulate immune responses in pts post-CAR T cell therapy.
Methods: Peripheral blood samples were collected from pts receiving IBER (1.6mg)+dexamethasone (DEX) prior to Tx initiation and after 1 cycle in the CC-220-MM-001 (NCT02773030) trial. The functional effect of IBER+DEX on anti-BCMA CAR T cells before and after Tx was assessed in 7 pts by comparing proliferation, phenotypic makeup, antigen-specific cytokine production, and cytotoxic activity. Results were validated with ex vivo Tx of 4 samples from healthy volunteers. Immune activation induced by IBER+DEX was also assessed by flow cytometry in 17 pts previously treated with CAR T cell therapy.
Results: IBER+DEX Tx of pts with RRMM increased percent of CD4+ T cells, proportion of central and effector memory T cells, and T cell-expressing activation markers (OX40, CD28, CD38), and decreased proportion of TEMRA (terminally differentiated) T cells in CAR T cell production. CAR T cells from pts on IBER+DEX Tx had higher proliferation rates, increased proportion of CD4+ and HLA-DR+ cells, and decreased proportion of exhausted (PD1+, TIM3+, TIGIT+) cells. Stimulation of CAR T cells produced from pts on IBER+DEX Tx lead to greater TNFα and granzyme-B levels, and significantly more efficiency in killing MM cells than CAR T cells produced pre-Tx. Ex vivo Tx of healthy T cells with IBER increased the proportion of memory CAR T cells and enhanced CAR T cell expansion and functionality. Lastly, IBER+DEX Tx of pts with MM previously exposed to CAR T cell therapy increased T and NK cell proliferation and shifted T cells from a naive to an activated effector memory phenotype.
Conclusions: IBER+DEX Tx enhanced the proliferation, expansion, and functionality of manufactured CAR T cells in pts with RRMM. Our findings suggest that Tx with IBER prior to apheresis improves expansion, manufacturing success, and potency of CAR T cells. Moreover, IBER remains immunostimulatory post-CAR T cell infusion, suggesting IBER may provide benefit as post-CAR T cell therapy maintenance. Our results provide rationale for IBER as an adjunctive Tx to CAR T cell therapy, supporting further exploration of the effects of IBER Tx peri-CAR T cell apheresis and post-CAR T cell infusion to improve outcomes for pts with MM in clinical practice. This abstract was accepted and previously presented at EHA2025. All rights reserved. AA & MA contributed equally.
