Cellular and T cell engager Immunotherapy
Category: Cellular and T cell engager Immunotherapy
Non-linear impact of BMI on anti-BCMA CAR-T outcomes: Overweight paradox in myeloma
(PA-015) Non-linear Impact of BMI on Anti-bcma CAR-T Outcomes: Overweight Paradox in Myeloma

Diana Cirstea, MD
Medical Oncologist
Center for Multiple Myeloma, Massachusetts General Hospital, Harvard Medical School
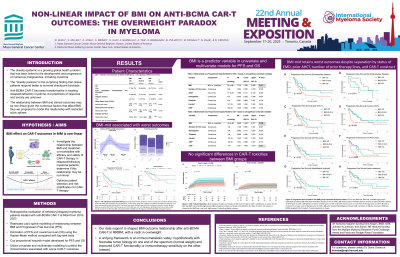
Anti-B cell maturation antigen (BCMA) chimeric antigen receptor T cell therapies (CAR-T) have been transformative in relapsed/refractory multiple myeloma (RR MM), yet predictors of response and toxicity remain incompletely understood. Obesity and metabolic co-morbidities may influence the outcomes via immune dysregulation and therapy tolerance. In this study, we investigated the relationship between BMI, and metabolic co-morbidities with efficacy and safety post-CAR-T. Our findings aim to optimize patient selection and risk stratification in this vulnerable population.
Methods:
We retrospectively evaluated a cohort of patients treated with anti-BCMA CAR-T for RR MM at Massachusetts General Hospital from 2016 – 2023. We utilized restricted cubic spline modeling to evaluate the relationship between BMI and progression free survival (PFS). PFS and overall survival (OS) were estimated using the Kaplan-Meier method and compared with log-rank tests. Multivariate Cox proportional hazards models for PFS and OS were utilized to predict factors associated with CAR-T outcomes.
Results:
Cubic spline modeling revealed a non-linear association between BMI and CAR-T outcomes. Overweight patients (BMI 25.0-29.9 kg/m2) had significantly worse PFS compared to normal weight ( < 25.0 kg/m2) and obese (≥30.0 kg/m2) cohorts: 12-month PFS was 51.9%, 28.8%, and 62.6% for normal-weight, overweight, and obese groups, respectively (P < 0.001). Similarly, 12-month OS was 82.9%, 61.4% and 84.2% (P=0.006), while the complete response rate was 42.9%, 36.4% and 56.1% (P=0.185) for normal-weight, overweight, and obese groups. Rates of cytokine release syndrome (CRS), immune effector cell neurotoxicity syndrome (ICANS), and inflammatory markers (ferritin, CRP, or LDH) were similar between all groups. Multivariate analyses adjusting for significant univariate factors (prior lines of therapy, transplant history, pre-lymphodepletion hemoglobin, platelets, ferritin, and CRP) confirmed superior PFS and OS in both normal-weight and obese patients compared to overweight patients.
Conclusions:
Our findings of a U-shaped BMI-survival relationship in MM patients treated with CAR-T challenges conventional linear analyses, suggesting that metabolic dysregulation may differentially impact CAR-T efficacy at different BMIs. We found a robust effect of BMI on CAR-T outcomes, with overweight BMI patients having worse outcomes than those with normal or obese BMIs. Given that obesity and metabolic disorders affect >40% of the global population and overweight/obesity rates continue to rise, understanding modifiable risk factors that can refine patient selection, or affect toxicity mitigation, and supportive care strategies for CAR-T recipients is critical.
