Cellular and T cell engager Immunotherapy
Category: Cellular and T cell engager Immunotherapy
Talquetamab in Brazil: Initial Experience and Outcomes Within an Expanded Access Program
(PA-083) Talquetamab in Brazil: Initial Experience and Outcomes Within an Expanded Access Program
.jpeg.jpg)
Fernando V. Pericole, MD, PhD
Assistant physician
Hemocentro Unicamp
Talquetamab (TAL) was approved in Brazil in 2024. Prior to this approval, Brazilian Expanded Access Program (EAP) enabled access to TAL in 2023 for patients with prior exposure to the three main classes of therapies (IMiDs, proteasome inhibitors, and anti-CD38 antibodies). This report presents real-world outcomes from patients treated under this EAP.
Methods:
We conducted a retrospective study of Brazilian patients enrolled in the TAL EAP. The study assessed safety and efficacy for patients from 8 independent Brazilian oncology centers. Dose schedule (0.4mg/kg weekly vs 0.8mg/kg every other week) was determined by physician's choice.
Results:
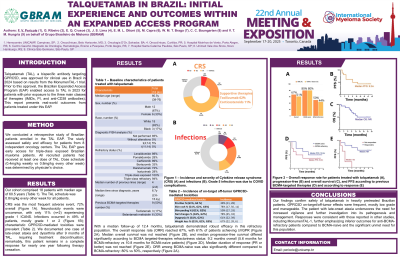
Our cohort comprised 18 patients (12M, 6F), median age of 60.5y (38-76), and median of 4 prior lines of therapy (2-8). All patients were triple-class exposed, with 94% of triple-class refractory. Notably, 33% (n=6) had received belantamab mafodotin, and 17% (n=3) had prior teclistamab exposure. Therefore, 50% of patients were BCMA-targeted therapies refractory as their last therapy. The TAL schedule was 0.8mg/kg Q2W for all patients. The safety profile revealed CRS as the most frequent AE, occurring in 72% of patients (gr1:44%, gr2:23%, gr3:5%), leading to tocilizumab in 62% of cases and fully resolved. Neurotoxicity events were uncommon, with only 11% (n=2) grade 1 ICANS, also resolved. Infections occurred in 49% of patients (gr1: 12%, gr2: 19%, gr3: 10%, gr4: 7% and 1 death due to COVID-19). We documented one case of late-onset ataxia and dysarthria after 9 months of TAL, requiring treatment discontinuation; remarkably, this patient remains in CR for nearly one year following therapy cessation. Characteristic GPRC5D-mediated toxicities were prevalent, including skin changes (78%; pruritus in 39%), dysgeusia (61%), weight loss in 67% of patients (gr1: 22%, gr2: 39%, and gr3: 6%), and nail abnormalities (78%).
With a median follow-up of 12.4 months, talquetamab demonstrated efficacy in this refractory population. The overall response rate (ORR) was 67%, with 61% of patients achieving ≥VGPR. Median OS was 13m, and median PFS differed significantly according to BCMA targeted-therapies refractoriness status: 9.2 months overall (5.6m for BCMA-refractory vs 10.8m for BCMA-naive patients). Median duration of response for responders (PR or better) was not reached. ORR among BCMA-naive was also significantly different compared to BCMA-refractory: 80% vs 50%, respectively.
Conclusions:
Our findings confirm the safety of talquetamab in heavily pretreated Brazilian patients. Notably, GPRC5D on-target/off-tumor effects were frequent, mostly low grade and manageable, consistent with published data. The patient with late-onset ataxia underscores the need for increased vigilance and further investigation into its pathogenesis and management. Responses were consistent with those reported in other studies, including MonumenTAL-1, further emphasizing the suboptimal outcomes for anti-BCMA-refractory patients and the significant unmet need for this population.
