Myeloma Genomics and Microenvironment and immune profiling
Category: Myeloma Genomics and Microenvironment and immune profiling
Referral of Select Multiple Myeloma Patients for Genetic Evaluation Leads to 5-Fold Increase in Pathogenic Germline Variant Detection
(PA-224) Referral of Select Multiple Myeloma Patients for Genetic Evaluation Leads to 5-Fold Increase in Pathogenic Germline Variant Detection

Maria Victoria del Rosal, MD (she/her/hers)
Research Associate
Department of Medicine, Hematology and Medical Oncology, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, NY
Methods:
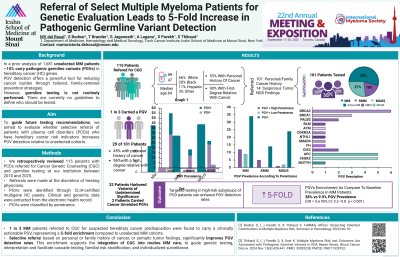
We retrospectively reviewed patients with PCDs referred for genetic counseling & germline testing at our institution between 2010 & 2025. Referrals were made at the discretion of treating physicians, based on clinical suspicion due to personal or family history of cancer and/or the presence of mutations in tumor NGS suggestive of germline origin. PGVs were identified through CLIA-certified multigene HC panels. Clinical & genomic data were extracted from the electronic health record. The primary outcome was PGV detection rate. 1 in 3 MM patients referred for suspected cancer predisposition carried a clinically actionable PGV, representing a fivefold enrichment over unselected MM cases. These findings highlight the feasibility & clinical utility of targeted germline screening in MM. A prospective study is underway to inform future testing guidelines.
Results: 115 PCD patients had a genetic evaluation by a certified genetic counselor. 101 were referred based on personal/family cancer history & 14 due to suspicious tumor NGS findings. Median age at referral was 64 (range 22–88) & 41% were male. 66% were White, 20% Black, 11% Hispanic & 3% Other. 61 patients (53%) had a personal history of other cancers & 104 (90%) had a first- or second-degree relative with cancer. 100 referred patients completed germline testing (87%), including 55 with MM, 18 smoldering MM (SMM) & 27 MGUS. 29 tested patients (29%) carried PGVs associated with HC: 20 MM (36% of all MM cases), 5 SMM (28% of all SMM cases) & 3 MGUS (11% of all MGUS cases). Compared to our previously published unselected MM cohort with a 9.9% PGV prevalence, the 36% detection rate in this selected MM subgroup represents a highly significant enrichment (OR 5.2, 95% CI 2.9–9.2, p=2.8×10⁻⁷). 18 of 29 PGVs were in high-penetrance HC genes, including BRCA2 (6), BRCA1 (3), PALB2 (2) & 7 others. The remaining 11 PGVs were low-penetrance founder variants in APC (5), CHEK2 (5) & MUTYH (1). 2 additional MM patients, not included in above calculations, were incidentally found to carry clinically actionable PGVs unrelated to cancer risk (long QT syndrome & hypertrophic cardiomyopathy). 13 PGV carriers (45%) had a personal history of cancer, most commonly colon (4), breast (3) & thyroid (3). 28 PGV carriers had a first-degree relative with cancer (97%), most frequently breast (13), gynecologic (9) & pancreatic (7).
Conclusions:
