MRD and Biomarkers
Category: MRD and Biomarkers
The Role of CHIP Mutations in Plasma Cell Dyscrasias: Implications for Clonal Evolution and Risk Stratification
(PA-172) The Role of CHIP Mutations in Plasma Cell Dyscrasias: Implications for Clonal Evolution and Risk Stratification

Kristin Ezell, MD
Resident Physician
University of Connecticut
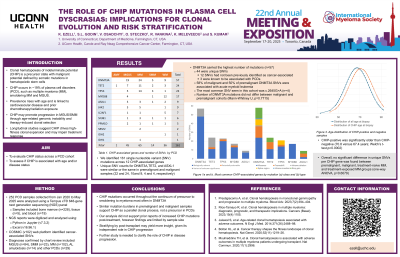
Introduction: Clonal hematopoiesis of indeterminate potential (CHIP) is a premalignant state defined by somatic mutations in hematopoietic stem cells. DNMT3A, TET2, and ASXL1 are common mutations. CHIP occurs in ~19% of plasma cell disorders (PCD), such as multiple myeloma (MM), smoldering MM and MGUS. Prevalence rises with age and is linked to cardiovascular disease and prior chemotherapy/radiation exposure and CHIP may promote progression in MGUS/SMM through age-related genomic instability and therapy-induced clonal selection. In MM, CHIP may predict poorer outcomes, particularly post-autologous stem cell transplant (ASCT). High-throughput single-cell sequencing has detected CHIP mutations in nearly half of ASCT patients. Longitudinal studies suggest CHIP drives high-fitness clonal expansion and may impair treatment response.
Methods: We analyzed genomic variants from 252 PCD samples using a Tempus xT® 648-gene next generation sequencing (NGS) panel. Samples included bone marrow (n=228), tissue (n=5), and blood (n=19), collected from Jan 2020 to May 2025. NGS reports were digitized and analyzed using Python in Jupyter (v7.3.2) and Excel (v16.96.1). COSMIC (v102) web platform identified cancer-associated SNVs. Diagnoses confirmed by chart review included MGUS (n=84), SMM (n=23), MM (n=102), AL amyloidosis (n=14) and other PCDs (n=29).
Results:
We identified 161 mutations across 12 CHIP-associated genes. DNMT3A carried the highest number of mutations (n=57), 44 of which were unique single nucleotide variants (SNV). 12 SNVs had not been previously identified as cancer-associated, and only 3 were known to be associated with PCDs. 58% of malignant and 50% of premalignant DNMT3A SNVs were associated with acute myeloid leukemia. The most common SNV seen in this cohort was c.2645G >A (n=4). Number of DNMT3A mutations did not differ between malignant and premalignant cohorts (Mann-Whitney U, p=0.7715). CHIP-positive was older than CHIP-negative in the overall cohort (70.8 versus 67.4 years; Welch’s t-test, p=0.0002), and distribution curves highlight this shift. Unique SNV counts for DNMT3A, TET2, and ASXL1 were similar or the same in premalignant and malignant samples (22 and 24, 10 and 9, 4 and 4, respectively). Overall, no significant difference in unique SNVs per CHIP gene was found between premalignant, malignant, treatment-naive MM, and treatment-exposed MM groups (one-way ANOVA, p=0.6079).
Conclusions: CHIP mutations occurred across all disease states, most often in DNMT3A. Similar mutation burdens in premalignant and malignant samples support CHIP as a parallel clonal process, not a precursor. Our analysis did not support prior reports of increased CHIP mutations post-treatment; however, it is limited by a sample size. Stratifying by post-ASCT may yield more insight, given its role as a potential contributor to disease complexity and resistance. Further study is needed to clarify the role of CHIP in disease progression.
