Cellular and T cell engager Immunotherapy
Category: Cellular and T cell engager Immunotherapy
Evaluation of sequential Absolute Lymphocyte Count (ALC) in patients with relapsed multiple myeloma (RRMM) with Cranial Nerve Palsies (CNPs) post Ciltacabtagene-autoleucel (cilta-cel)
(PA-026) Evaluation of Sequential Absolute Lymphocyte Count (ALC) in Patients with Relapsed Multiple Myeloma (RRMM) with Cranial Nerve Palsies (CNPs) Post Ciltacabtagene-autoleucel (cilta-cel)

Karla J. Feliciano Salva, MD (she/her/hers)
Blood and Marrow Transplant and Cellular Immunotherapy Fellow
Department of Blood & Marrow Transplant and Cellular Immunotherapy (BMT CI), Moffitt Cancer Center
Cilta-cel is an FDA-approved CAR T-cell therapy targeting BCMA, for patients with RRMM after at least one prior therapy including a PI and IMID, or after four prior therapies including a PI, IMID, and CD38 antibody. Approval was based on the Cartitude 1 and 4 studies showing strong efficacy (Martin et al., 2023; San Miguel et al., 2023). Neurotoxicity occurs in up to 20% of patients, including ICANS and delayed effects like movement/neurocognitive toxicities (MNTs) and CNPs. MNTs are linked to high CAR T-cell expansion, measured by max ALC (Cohen et al., 2022), but factors associated with CNPs remain unclear. We aim to study baseline features and sequential ALC in these patients.
Methods:
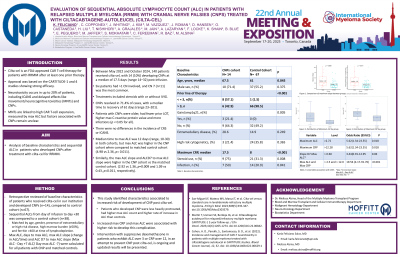
We retrospectively reviewed the baseline characteristics of patients who received cilta-cel in our institution and developed CNPs (n=14), compared to control cohort (n=67). We examined sequential ALCs from day of cilta-cel infusion to day +30 and compared to a control cohort (n=38), matched by age, gender, presence of extramedullary or high-risk disease, high marrow burden (≥50%), and ferritin >400 at time of lymphodepletion. Max ALC, days to max ALC, max ALC slope (change in ALC/time) and ALC D7 to max ALC slope (Max ALC - Day +7 ALC/ Day max ALC -7) were calculated for all patients with CNP and matched controls.
Results:
Between May 2022 and October 2024, 140 patients received cilta-cel, with 14 (10%) developing CNPs at a median of 17.5 days (range 14–32) post-infusion. Six patients had >1 CN involved, and CN 7 (n=11) was the most common. Treatments included steroids with or without IVIG. CNPs resolved in 71.4% of cases, with a median time to recovery of 61 days (range 23–201). Patients with CNPs were older, had fewer prior LOT, higher max C-reactive protein value and more infections (p < 0.05 for all). There were no differences in the incidence of CRS or ICANS. Median time to max ALC was 12 days (range, 10-30) in both cohorts, but max ALC was higher in the CNP cohort when compared to matched control cohort (4.98 vs 2.36, p= 0.011). Similarly, the max ALC slope and ALCD7 to max ALC slope were higher in the CNP cohort vs the matched control cohort. (2.92 vs 1.36, p=0.009 and 1.09 vs 0.43, p=0.011, respectively).
Conclusions:
This study identified characteristics associated to increased risk of development of CNP post cilta-cel and showed that increased max CRP and max ALC were associated with higher risk to develop this complication. Intervention with suppressive dexamethasone in patients who exhibit ALC over 3 or CRP over 12, in an attempt to prevent CNP post cilta-cel, is ongoing and updated results will be presented.
