Treatment of Relapsed/Refractory Myeloma (excluding T-cell redirection therapy)
Category: Treatment of Relapsed/Refractory Myeloma (excluding T-cell redirection therapy)
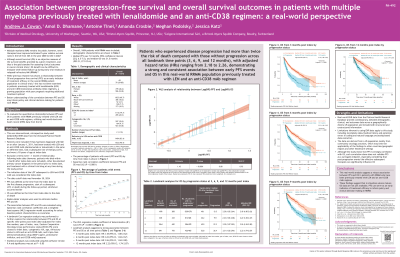
ASSOCIATION BETWEEN PROGRESSION-FREE SURVIVAL AND OVERALL SURVIVAL OUTCOMES IN PATIENTS WITH MULTIPLE MYELOMA PREVIOUSLY TREATED WITH LENALIDOMIDE AND AN ANTI-CD38 REGIMEN: A REAL-WORLD PERSPECTIVE
(PA-492) Association Between Progression-Free Survival and Overall Survival Outcomes in Patients with Multiple Myeloma Previously Treated with Lenalidomide and an ANTI-CD38 Regimen: A Real-World Perspective
- AC
Andrew J. Cowan, MD
Associate Professor of Medicine
Division of Medical Oncology, University of Washington
Introduction:
Progression-free survival (PFS) and overall survival (OS) are crucial endpoints in clinical trials for relapsed/refractory multiple myeloma (RRMM). However, due to the extended duration of OS, mature OS data is often unavailable during early trial readouts. In such scenarios, PFS can serve as an early indicator of treatment efficacy. While previous research has investigated the relationship between PFS and OS in the overall RRMM patient population in clinical trials, this relationship has not been examined in patients previously treated with lenalidomide (LEN) and anti-CD38 regimens. This study aims to evaluate the quantitative relationship between PFS and OS in patients with RRMM previously treated with LEN and an anti-CD38 regimen, utilizing real-world electronic heath record (EHR)-data from the United States.
Methods:
This non-interventional, retrospective study used the US-based, EHR-derived deidentified Flatiron Health Research Database. Patients diagnosed with MM on or after January 1, 2011, who were treated with LEN and an anti-CD38 monoclonal antibody, and had a subsequent line of therapy (LOT), were included. The initiation date of this subsequent LOT was considered the index date; the study end date was November 30, 2024. SAS v9.4 was used to assess PFS and OS outcomes post-index. PFS was defined as the time from the index date to the first disease progression, start of a subsequent LOT, or death during the follow-up period, whichever occurred earlier. Kaplan-Meier analyses were used to estimate median PFS and OS. The association between PFS and OS was evaluated using Spearman's rank correlation coefficient, weighted least squares (WLS) regression model, and multivariable Cox proportional hazards regression models. The multivariable model accounted for select baseline characteristics as covariates. A landmark analysis was performed to further explore the relationship between PFS and OS at specific time points. Statistical significance was set at a P value of < 0.05.
Results:
A total of 1,684 patients with RRMM were included in the study. The median age was 69 years (range, 29-85), with 53.8% male. The median PFS was 7.0 months (95% confidence interval [CI], 6.5–7.5 months), and the median OS was 31.5 months (95% CI, 27.9–35.4 months). Spearman’s rank correlation coefficient between PFS and OS was 0.62 (P < 0.0001). The WLS regression model R² value was 0.93 (P < 0.0001). Landmark analyses demonstrated a strong association between PFS and OS at 3 months (HR, 2.18; 95% CI, 1.80–2.62), 6 months (HR, 2.22; 95% CI, 1.84–2.67), 9 months (HR, 2.26; 95% CI, 1.84–2.80), and 12 months (HR, 2.22; 95% CI, 1.74–2.87).
Conclusions:
This real-world analysis reveals a robust association between PFS and OS in patients with RRMM who have been previously treated with LEN and an anti-CD38 regimen. In situations where mature OS data are not yet available, these findings suggest that PFS can serve as an early indicator of treatment efficacy to inform policy and decision-making in healthcare.
