Cellular and T cell engager Immunotherapy
Category: Cellular and T cell engager Immunotherapy
Long-Term Follow-Up from MajesTEC-1 China Cohort of Teclistamab in Patients with Relapsed/Refractory Multiple Myeloma: Efficacy Updates, Infection Profile and Immunoglobulin Usage
(PA-101) Long-Term Follow-Up from MajesTEC-1 China Cohort of Teclistamab in Patients with Relapsed/Refractory Multiple Myeloma: Efficacy Updates, Infection Profile and Immunoglobulin Usage

Zhen Cai, MD (she/her/hers)
Chief Physician
Bone Marrow Transplantation Center, The First Affiliated Hospital, School of Medicine, Zhejiang University
Introduction:
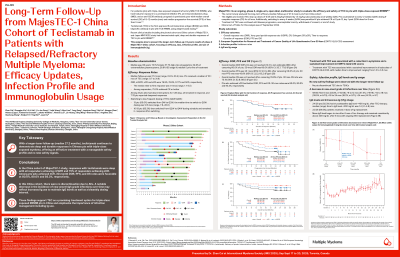
Teclistamab (tec) is the first approved BCMA×CD3 bispecific antibody in patients (pts) with triple-class exposed relapsed/refractory multiple myeloma (RRMM) using weight-based dosing. In trials and real-world setting, tec has shown rapid, deep, and durable responses. We report longer-term follow-up (FU) results of Phase 2 MajesTEC-1 China cohort on efficacy, infection profile and immunoglobulin (Ig) usage.
Methods:
MajesTEC-1 China cohort enrolled 26 RRMM pts who had received ≥3 prior lines of therapy including a PI, IMiD and anti-CD38 mAb. After providing informed consent, pts received tec 1.5 mg/kg subcutaneous QW preceded by step-up dosing, pts could switch to less frequent dosing if achieved ≥CR for ≥6 mos.
Results:
As of Sep 27, 2024, mFU was 27.2 months (mos) (range: 0.8 to 33.4). Pts received a median of 10.1 mos of tec (range: 0.7 to 31.9). ORR, ≥VGPR, ≥CR and sCR were 76.9%, 76.9%, 57.7% and 50%, respectively. Among 13 pts (50%) who switched from QW to Q2W, median time to switch was 12.6 mos (range: 7.9 to 20.0) and 10/13 then moved to Q4W and remained in response. Overall median DOR was not reached (30-mos DOR: 60.0%); median PFS was 25.1 mos (30-mos PFS: 43.6%); and median OS was not reached (30-mos OS: 75.1%). Pts who achieved ≥CR had better DOR, PFS and OS: 30-mos rates were 80.0%, 80.0% and 93.3%, respectively. Treatment with tec was associated with a sustained improvement in all subscales of EORTC-QLQ C30, with median time to improvement ranging from 1.3 to 3.5 mos.
In terms of infection profile, the incidence of new-onset grade ≥3 infections were more frequent within the first 6 mos of tec therapy and decreased over time: 53.8% (14/26) within first 6 mos, 47.4% (9/19) within >6 to 12 mos, 38.5% (5/13) within >12 to 18 mos, 18.2% (2/11) within >18 to 24 mos, and 14.3% (1/7) >24 mos. 24 pts (92.3%) had ≥1 postbaseline IgG level < 400mg/dL after tec therapy; median time to IgG < 400mg/dL was 1.4 mos (range: 1.1 to 4.8), and 22 pts (84.6%) received ≥ 1 dose of Ig replacement. Mean IgG level began to rise after 6 mos of tec therapy and remained consistently above 400 mg/dL after 8 mos.
For neutropenia, grade ≥3 neutropenia was reported in 20 pts (76.9%). No pts discontinued tec due to neutropenia. No febrile neutropenia was reported. 23 pts (88.5%) received colony-stimulating factors, and the incidence of new-onset grade ≥3 neutropenia decreased over time.
| mFU | 27.2mos |
| ORR | 76.9% |
| ≥CR | 57.7% |
| Median DOR | NR |
| 60.0% |
| 80.0% |
| Median PFS | 25.1mos |
| 43.6% |
| 80.0% |
| Median OS | NR |
| 75.1% |
| 93.3% |
Conclusions: With a mFU of 27.2 mos, tec consistently showed deep and durable responses. The overall safety profile remained consistent with a notable decrease in incidence of new onset high grade infections over time. These data are consistent with the MajesTEC-1 pivotal cohort and support tec as a promising treatment option for pts with triple‐class exposed MM in China.
