Treatment of Relapsed/Refractory Myeloma (excluding T-cell redirection therapy)
Category: Treatment of Relapsed/Refractory Myeloma (excluding T-cell redirection therapy)
Isatuximab Subcutaneous (Isa SC) via On-Body Injector (OBI) or Manual Injection for Relapsed/Refractory Multiple Myeloma (RRMM): Patient Experience from the Phase 2 IZALCO Study
(PA-489) Isatuximab Subcutaneous (Isa SC) via On-Body Injector (OBI) or Manual Injection for Relapsed/Refractory Multiple Myeloma (RRMM): Patient Experience from the Phase 2 IZALCO Study

Vania Hungria, MD, PhD (she/her/hers)
Professor
Clinica São Germano
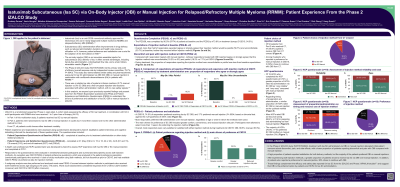
Improved drug delivery methods are needed to reduce patient burden and increase treatment adherence in MM. The OBI is a wearable injector applied by a healthcare professional for delivery of Isa SC. The Phase 2 IZALCO study (NCT05704049) met its primary endpoint, demonstrating efficacy (ORR, 79.7%), safety and similar pharmacokinetic exposure for Isa SC administered via OBI or manual injection in combination with carfilzomib-dexamethasone in RRMM patients. There was a notable low rate of infusion reactions, with no new safety signals except for few low-grade injection site reactions associated with the SC route. On the key secondary endpoint, 74.5% of patients preferred receiving Isa SC by OBI and 17% preferred manual injection (p=0.0004). Here we report a detailed evaluation of patient experience and satisfaction with Isa SC OBI or manual injection in IZALCO.
Methods:
In Part 1 of this multicenter study, 8 of 74 patients received Isa SC by manual injection. In Part 2, patients were randomized to Isa SC OBI or manual injection in cycles (C)1-3 and then crossed over to the other administration method for C4-6. From C7, all patients could choose either treatment modality. Patient expectations were assessed before treatment using the Patient Expectation Questionnaire (PEQ) at baseline and patient experience was assessed during all treatment cycles with the Patient Experience and Satisfaction Questionnaire (PESQ v.2). Quality of life was evaluated with the EORTC-QLQ-C30 questionnaire at C1/day (D)1 or 2, D15 or D16 of subsequent cycles and end of treatment.
Results:
The PEQ was completed by 90.5% (67/74) of patients at baseline and the PESQ by ≥73.8% on treatment (except C12D15, 54.5%). During treatment, the proportion of patients reporting discomfort (OBI: 0-13.3%; manual: 3.4-18.5%), pain (OBI: 0-22.7%; manual: 6.9-20.0%) and side effects from injection method (OBI: 0-12.5%; manual: 3.4-15.4%) was lower than pre-treatment expectations of discomfort (53.7%), pain (56.7%) and side effects (53.7%). Fewer patients treated with OBI vs manual injection reported discomfort, pain and side effects from injection method at most time points. During treatment, most patients were satisfied with either injection method (OBI: 80.6-97.1%; manual: 81.5-95.1%) and would recommend the medication to other patients (OBI: 87.0-100%; manual: 89.6-100%). Global health status on the EORTC-QLQ-C30 was maintained relative to baseline, with no notable difference observed between delivery methods. 89.4% of the C6D15 PESQ respondents opted for Isa OBI at C7 onward.
Conclusions:
In IZALCO, treatment with Isa SC administered via OBI or manual injection alleviated initial patient concerns about discomfort, pain and side effects, and resulted in a high proportion of patients reporting satisfaction. These findings complement the patient preference data reported for Isa OBI vs manual injection and suggest that the OBI is a convenient option for Isa SC administration.
Funding: Sanofi.
