Cellular and T cell engager Immunotherapy
Category: Cellular and T cell engager Immunotherapy
Efficacy of subsequent T Cell Engagers following BCMA CAR-T therapy in Patients with Relapsed/Refractory Multiple Myeloma: A Systematic Review and Meta-Analysis
(PA-100) Efficacy of Subsequent T Cell Engagers Following BCMA CAR-T Therapy in Patients with Relapsed/refractory Multiple Myeloma: A Systematic Review and Meta-analysis
.jpg)
Nikhil Vojjala, MD
PGY 3 Chief Resident
Trinity Health, WSUSOM
B-cell maturation antigen (BCMA)-targeted therapies, including CAR-T, bispecific antibodies (BsAbs), and antibody-drug conjugates (ADCs), have significantly advanced the treatment of relapsed/refractory multiple myeloma (RRMM). However, disease progression after BCMA-therapies remains a critical challenge, and the response to subsequent salvage treatments in this setting is unclear. The purpose of this study is to evaluate the pooled objective response rates (ORR) of various BCMA and non-BCMA targeted therapies administered following prior BCMA CAR-T therapy.
Methods:
A systematic literature search was conducted to identify studies that investigated sequential therapies in RRMM patients using PubMed, ASH 2024, ASCO 2025, and EHA 2025 abstracts using the search terms (Multiple Myeloma) AND (Bispecific antibodies) OR (CAR-T cell therapy) OR (BCMA ADC) AND (Sequential therapies). Our search yielded 492 studies in PubMed, and 19 studies were included in the final analysis. Additionally, 5 abstracts from ASH and 7 abstracts from ASCO were included in the final analysis.
We conducted a single-arm proportion meta-analysis for outcomes in various categories. A random-effects model was used for outcomes with high heterogeneity (defined by an I² statistic greater than 25% and a p-value in the Cochrane test greater than 0.1). Otherwise, a fixed effects model was used. Categorical outcomes were summarized by pooled proportion with 95% CI. P < 0.05 was considered statistically significant. All the analysis was carried out using R Studio desktop version 4.4.2. The primary outcome evaluated is ORR.
Results:
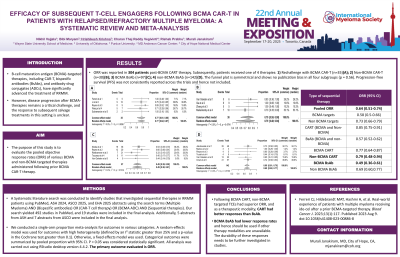
ORR was reported in 304 patients post-BCMA CART therapy. Subsequently, patients received one of 4 therapies: 1) Rechallenge with BCMA CAR-T (n=35); 2) Non-BCMA CAR-T (n=30); 3) BCMA BsAb (n=97); 4) non-BCMA BsAb (n=142). The pooled ORR is 0.64 (95% CI: 0.51-0.74). The ORR rates are 77%, 79%, 49%, and 69%, respectively, in four sub-groups. On further analysis, there was significantly higher ORR with non-BCMA targets as compared to BCMA targets (non-BCMA CAR-T plus BsAb vs BCMA CAR-T plus BsAb) [ORR=0.73 (0.66- 0.79) vs ORR=0.58 (0.50- 0.66), p= 0.006]. Similar trends of higher ORR were noticed with CAR-T compared to Bispecific antibodies, irrespective of the target [ORR=0.85 (0.75- 0.91) vs ORR=0.57 (0.52 to 0.62), p< 0.001]. Additionally, we analyzed the cohort that received any prior BCMA followed by BCMA BsAbs, and the ORR was 51%. The funnel plot is symmetrical and shows no publication bias in all 4 subgroups (p=0.56). Progression-free survival (PFS) was not consistently reported across the trials and hence not included.
Conclusions:
Following BCMA CART, non-BCMA targeted TCEs had superior ORR, and as a therapeutic modality, CART had better responses than BsAb. BCMA BsAb had lowest response rates and hence should be used only if other therapy modalities are unavailable. The durability of these responses needs to be determined in further studies.
