Treatment of Relapsed/Refractory Myeloma (excluding T-cell redirection therapy)
Category: Treatment of Relapsed/Refractory Myeloma (excluding T-cell redirection therapy)
Real-world (RW) treatment (Tx) patterns and clinical outcomes in patients (pts) with relapsed/refractory multiple myeloma (RRMM) with 2–4 lines of treatment: data from the US Flatiron database
(PA-490) Real-world (RW) Treatment (Tx) Patterns and Clinical Outcomes in Patients (pts) with Relapsed/refractory Multiple Myeloma (RRMM) with 2–4 Lines of Treatment: Data from the US Flatiron Database

Maria J. Nieto, MD
Assistant Professor/Attending Hematology Oncology
Donald and Barbara Zucker School of Medicine at Hofstra/ R.J. Zuckerberg Cancer Center
Methods:
This study used electronic health records from a longitudinal, nationwide research database (Flatiron Health). Eligible pts were ≥ 18 y, diagnosed with RRMM from Jan 1, 2011 through Dec 31, 2024, and received 2L Tx or progressed from 2L to 3L/4L Tx. Pts who initiated 2L Tx before Jan 1, 2019 were excluded. Index date was the start date of each LOT. Frailty was defined based on a simplified score. Descriptive statistics were used to describe pt characteristics and Tx patterns by LOT. Kaplan–Meier curves were used to describe clinical outcomes from index date including progression-free survival (PFS) and overall survival (OS).
Results:
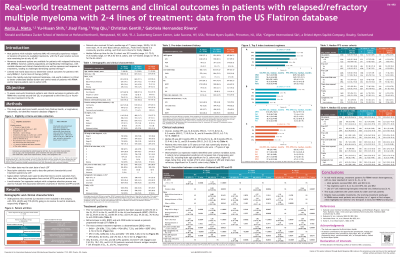
Overall, 3538 pts who received 2L Tx were included in this analysis; 1514 (42.8%) and 719 (20.3%) went on to receive 3L and 4L Tx, respectively. For pts who received 2L Tx, median age was 71 y (range, 28–85), median follow-up was 20.5 mo (range, 0.1–72.0), 53.3% were male, 19.1% were African American, 74.8% were treated in a community practice setting, and 39.6% were considered frail.
Prior to Tx initiation, 70.3% (2L), 83.1% (3L), and 89.2% (4L) of pts had been exposed to lenalidomide (LEN); 78.0% (2L), 84.5% (3L), and 87.1% (4L) to bortezomib (BORT); and 16.4% (2L), 54.2% (3L), and 76.4% (4L) to an anti-CD38 monoclonal antibody (aCD38).
Prior to LOT start, 46.5% (2L), 61.8% (3L), and 70.9% (4L) of pts were LEN-refractory; 46.4% (2L), 55.7% (3L), and 59.2% (4L) of pts were BORT-refractory; and 12.8% (2L), 46.2% (3L), and 68.2% (4L) of pts were aCD38-refractory.
The most common Tx regimens (± dexamethasone) used in 2L were daratumumab (DARA) + LEN (DRd, 7.2%), DARA + pomalidomide (DPd, 7.2%), and DARA + BORT (DVd, 6.1%); in 3L, DPd (11.0%), DARA (Dd, 6.3%), and DARA + carfilzomib (CFZ) (DKd, 5.6%); and in 4L, DPd (8.8%), Dd (5.0%), and CFZ (Kd, 4.7%).
Only 8, 22, and 27 pts received T-cell engagers and 9, 18, and 24 pts received CAR T cell therapies in 2L, 3L, and 4L, respectively.
Overall, median PFS was 12.8 mo (95% CI, 11.9–13.8) for 2L, 8.2 mo (95% CI, 7.3–8.9) for 3L, and 6.9 mo (95% CI, 6.2–7.9) for 4L; median OS was 60.5 mo (95% CI, 55.9–64.5) for 2L, 38.9 mo (95% CI, 33.9–46.1) for 3L, and 29.0 mo (95% CI, 24.3–32.3) for 4L. Pts who were older (≥ 75 y) or frail had numerically shorter 2L and 3L PFS and OS compared with pts who were < 75 y or non-frail.
Conclusions: There is no clear standard of care for pts with RRMM in RW clinical practice. Despite the availability of numerous Tx options, outcomes remain poor for pts, even in early-line RRMM. This study also highlights higher unmet needs for older and frail pts.
