Treatment of Newly Diagnosed Myeloma (excluding t-cell redirection therapy)
Category: Treatment of Newly Diagnosed Myeloma (excluding t-cell redirection therapy)
Race, Lenalidomide Dosing, and Survival in Newly Diagnosed Transplantation-Ineligible Multiple Myeloma Patients
(PA-395) Race, Lenalidomide Dosing, and Survival in Newly Diagnosed Transplantation-Ineligible Multiple Myeloma Patients

Armand Ghazanfar, PharmD
Pharmacy Student
Texas Tech University Health Sciences Center
Lenalidomide (LEN) is central to the management of newly diagnosed multiple myeloma (NDMM). However, LEN dosage reductions may occur, especially in transplantation-ineligible patients who are less fit. These reductions may occur differently in African American (AA) patients due to socioeconomic disparities or baseline neutropenia due to the Duffy-null phenotype. We sought to determine the prevalence and clinical impact of LEN dose reductions in AA patients using a large United States Veterans Affairs (VA) database.
Methods:
This was a retrospective VA cohort study of transplantation-ineligible patients with NDMM treated between Jan 2015 and Dec 2023. Cox proportional hazard models were constructed to determine the difference in LEN dose reductions and all-cause mortality within 5 years between dose-reduced and non-dose-reduced patients, adjusting for age, sex, race, ethnicity, ISS stage, and other covariates. Kaplan-Meier curves examined times to dose reduction and time to mortality.
Results:
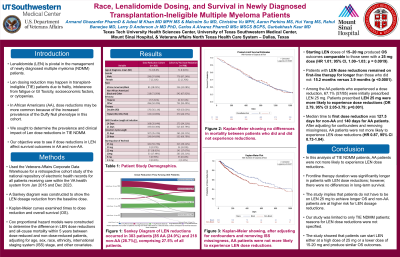
Of 1101 patients, the mean (SD) age at diagnosis was 72.1 (8.5) years; there were 341 (31.0%) AA patients. Starting LEN doses were 25 mg in 494 (44.9%), 20 mg in 33 (3.0%), and 15 mg in 198 (18.0%). Starting LEN doses of 15–20 mg produced overall survival (OS) outcomes comparable to those seen with a 25 mg dose (HR 1.01; 95% CI, 1.00–1.03; p = 0.0919). LEN reductions occurred in 303 patients [85 AA (24.9%) and 218 non-AA (28.7%)], comprising 27.5% of all patients. Patients with LEN dose reductions remained on first-line therapy for longer than those who did not: 15.2 months versus 3.9 months. Among the AA patients who experienced a dose reduction, 67.1% (57/85) were initially prescribed LEN 25 mg. Patients prescribed LEN 25 mg were more likely to experience dose reductions (OR 2.79, 95% CI 2.05-3.79; p< 0.001). The median time to first dose reduction was 127.5 days for non-AA and 140 days for AA patients. After adjusting for confounders and removing missingness, AA patients were not more likely to experience LEN dose reductions (HR 0.87, 95% CI 0.73-1.04). Patients older in age, higher starting doses, and neutropenia were more likely to experience reductions. There were no differences in mortality between patients who did and did not experience reductions (HR 0.84, 95% CI 0.68-1.04); instead, baseline thrombocytopenia, older age, and higher ISS Stage were associated with reduced OS.
Conclusions:
In this analysis of over 1000 transplantation-ineligible patients with NDMM, AA patients were not more likely to experience LEN dose reductions. Even after adjusting for race, patients who underwent a dose reduction showed no differences in outcomes. Frontline therapy duration was significantly longer in patients with LEN dose reductions; however, there were no differences in long-term survival.
