Myeloma Genomics and Microenvironment and immune profiling
Category: Myeloma Genomics and Microenvironment and immune profiling
Treatment of Plasma Cell Disorders Post Solid Organ Transplant – A Positive Single Center Experience using Immunomodulatory Drugs
(PA-264) Treatment of Plasma Cell Disorders Post Solid Organ Transplant – A Positive Single Center Experience using Immunomodulatory Drugs

Khalida B. Soki, MD (she/her/hers)
Transplant Nephrology Fellow
University of Alberta
Introduction: Immunomodulatory drugs (IMiDs) have significantly improved prognosis in the treatment of plasma cell disorders. There is reticence to prescribe IMiDs to solid organ transplant (SOT) recipients as the limited literature to date suggests that IMiDs carry a significant risk of solid organ transplant rejection (SOTr). We aim to review the outcomes of SOT recipients with plasma cell disorders treated with IMIDs at a single center.
Methods:
We conducted a retrospective chart review of all SOT recipients at our center, from 2016 to 2025, who were treated for plasma cell disorders. Data collected included transplant type, treatment details & patient & allograft outcomes.
Results:
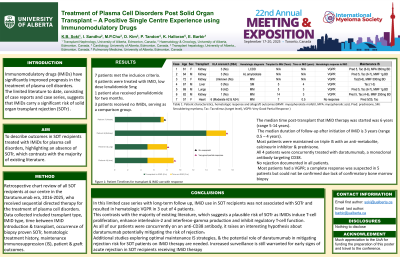
7 patients met the inclusion criteria: 4 received IMiD, lenalidomide 5mg, 1 patient also received pomalidomide & 3 patients received no IMiDs, serving as a comparison group. Clinical indications for IMiD included treatment of de novo disease, relapsed disease & maintenance therapy. Median time post-transplant to IMiD therapy initiation was 6 years (range 5-14 years). Median duration of follow-up after initiation of IMiD was 3 years (range 0.5–4 years). Most patients were maintained on triple IS. Of note, all 4 patients were concurrently treated with daratumumab, a monoclonal antibody targeting CD38. There was no rejection documented in all patients.
Case | Age | Sex | HLA Mismatch (DSA) | Haematological Diagnosis | Transplant to IMiD (Years) | Time on IMiD (Years) | Haematological response to IMiD | IS |
1 | 61 | F | 3 | LCDD | - | - | VGPR | Pred 5, Tac (6-8), MPA 360mg BD |
2 | 64 | M | 3 | AL Amyloidosis | - | - | VGPR | Pred 5, Tac (6-7), MMF 1g BD |
3 | 72 | F | Unknown | MM | - | - | VGPR | Tac (5-6), MMF 500mg BD |
4 | 67 | M | 0 | SM | 5 | 4 | VGPR | Tac (1-3) |
5 | 69 | M | 6 (A2) | MM | 5 | 3 | VGPR | Pred 5, Tac (6-7), MMF 1g BD |
6 | 82 | M | 1 | MM | 15 | 3 | VGPR | Pred 5, Tac (4-6), MMF 250mg BD |
7 | 57 | F | 6 (Moderate A2, A24) | MM | 7 | 0.5 | No response | Pred 5/25, Tac |
Table 1: Patient characteristics, hematologic response & allograft outcomes (MMF: mycophenolate mofetil, MPA: mycophenolic acid, Pred: prednisone, SM: Smouldering myeloma, Tac: Tacrolimus (target level), VGPR: Very Good Partial Response)
Conclusions:
In this case series, IMiD use in SOT recipients was not associated with SOTr & resulted in hematologic VGPR in 3 out of 4 patients. This contrasts with the majority of existing literature, which suggests a risk of SOTr as IMIDs induce T-cell proliferation, IL-2 & IFN-γ production & inhibit regulatory T-cell function. As all of our patients were concurrently on an anti-CD38 antibody, it raises a hypothesis about daratumumab potentially mitigating the risk of rejection. A recent study demonstrated that anti-CD38 Felzartamab was effective in the treatment of antibody mediated rejection, & while T-cell mediated rejection (TCMR) has been primarily associated with IMiD use, in this study, the anti-CD38 did not induce TCMR.
Additional studies exploring optimal IS strategies & the potential role of daratumumab in mitigating rejection risk for SOT patients on IMiD therapy are needed.
